
DUCHENNE MUSCULAR DYSTROPHY (DMD)
What is it?
Duchenne Muscular Dystrophy is a genetic disorder that affects a recessive gene that is connected to the X cromossome.
DMD afects 20 to 30 children in every 100 000 births of the male sex. The symptoms manifest between the age of 3 and 7, initially under the form of muscular weakness in the pelvic zone and, later on, in the shoulders. Children with Duchenne Muscular Dystrophy have a near total lack of Dystrophin, an essential muscle protein, which is responsible for the maintenance of muscle cells' structure. As the disease evolves, the muscles increase in size, but the muscle tissue gets weaker. 90% of children with this disease suffer from weakness of the cardiac muscle, which may cause heart problems. The arms' and legs' muscles often suffer contractures around the articulations, so the affected person can't fully extend their elbows or knees.
Despite the fact that the female gender is a carrier of this anomalous gene, the majority of female DMD carriers are asymptomatic (carrier for a disease or infection but experiences no symptoms). Female carriers most likely won't suffer from the disease, because the normal X cromossome will compensate the genetic anomaly of the other X cromossome. However, 2-20% of all female carriers show evidence of muscle weakness. Any male that receives the affected X cromossome will suffer from the disease.

DMD is caused by mutations in the Dystrophin gene, one of the largest known genes in the human race, which occupies 0.1% of the total human genome. It's also responsible for Becker Muscular Dystrophy, a similar disease. This gene is responsible for encoding the protein in study, and mutations in this gene cause Dystrophin to miss function. (*)
The Role of Dystrophin
Dystrophin is a protein responsible for connecting the cytoskeleton of each muscle fiber to the underlying basal lamina, through a protein complex containing many subunits. Normal skeletal muscle tissue contains only small amounts of Dystrophin (about 0.002% of total muscle protein), but its absence (or abnormal expression) leads to the development of severe and currently incurable symptoms.
The lack of Dystrophin causes micro-holes in the sarcolemma, which allows an excessive amount of calcium to penetrate the sarcolemma, leading the cell to necrosis. The dead fibers will then be replaced by others. However, it gets to a point where the frequency of cell destruction is so big that the tissue will start to be replaced by adipose and connective tissue. It is at this stage where the debility starts and with time, it worsens progressively, becoming a factor that will decrease the life quality of the affected.
Diagnosis
The diagnosis is made when a progressive difficulty in the children's movements is detected. To confirm the diagnosis, doctors usually do a DNA test. The muscle-specific isoform of the Dystrophin gene is composed of 79 exons, and DNA testing and analysis can usually identify the specific type of mutation of the exon or exons that are affected (*). DNA testing confirms the diagnosis, in most cases.
If the DNA test fails, the doctor may then do a muscle biopsy for observation, which may reveal the presence of dead tissue (necrosis) and an increased size of the muscle fibers. As said before, in more advanced stages of this dystrophy, fat and other tissues replace the dead tissue, which can also be detected in the biopsy.
The diagnosis of DMD can also be made by exams that reveal extremely low levels of Dystrophin in the muscle.


Molecular Mecanisms of DMD
Duchenne Muscular Dystrophy is caused by the absence or by a decrease in the levels of the Dystrophin protein. This is due to a point mutation in the Dystrophin gene, which causes a defect in splicing, affecting its normal expression and protein production. If the nucleotide was not mutated, there would be no anomaly.
What is "Splicing"?
It is a mechanism of RNA processing that removes intron and ligates the tandemly arranged exons.

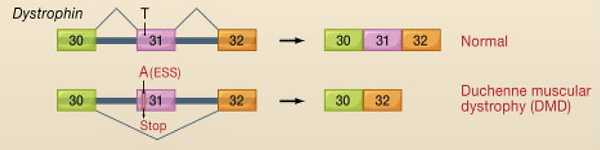
In DMD, the T-A substitution in exon 31 transforms the sequence into a regulatory sequence named Exon Splicing Silencer (ESS), which inhibits the splicing of the upstream intron. As a consequence, the gene is alternatively spliced. The transcription machinery splices exon 31 and surrounding introns, producing an mRNA that lacks exon 31, and therefore produces a defective protein.
Splicing in the Dystrophin Gene

Healthy skeletal muscle tissue

Skeletal muscle tissue with DMD
Adapted from Cooper et al (2009) Cell 136, 777–793
All images were taken from Google Images, unless said otherwise.
(*) Information taken from Wikipedia - 1st link in References